Custom Medical Battery Solutions for Top Medical Device Companies
Advanced lithium battery technology delivers unparalleled efficiency and convenience for everything from smartphones to portable medical devices. The advantages are significant. Using a lithium battery can reduce the weight of the device and extend the working time between charges, making it more useful for providing medical care. Medical Grade Lithium Batteries As you might expect, the safe integration of lithium batteries into the design is not only a major issue but also a challenge for medical device developers. Fortunately, many regulatory agencies such as UL, IEC, and FDA provide certification to regulate the safety of medical devices and the lithium batteries that power them. All certificates focus on the end user's security protection of the medical device in the target operating environment.
Medical device providers must comply with safety certificate guidelines
GREPOW believes that every medical device provider must comply with the safety certificate guidelines to provide users with safety and comfort. This is a problem because the current limited understanding of lithium battery technology and the risk of liability for lithium battery technology may pose greater risks to related equipment. Medical device developers need to fully understand and adopt the characteristics of lithium batteries in product design to minimize risk and exposure. For example, developers who must use rectangular lithium polymer batteries to install into their medical devices may experience inconsistent performance, expansion rates, and reliability issues after the use of Li-Po batteries. We have the knowledge to design cells to maintain good performance under these adverse conditions, reduce post-use expansion and improve reliability.

Medical device manufacturers should purchase certified lithium batteries
Medical grade batteries In order to comply with all medical certificates, lithium batteries must be produced by UL-certified factories. From chemical production to battery assembly and final testing, lithium battery production must be performed in a UL-certified facility. Any medical device manufacturer should not purchase lithium batteries from a factory that is not UL listed because they will not receive FDA approval. Therefore, to serve our customers, GREPOW has taken all necessary steps to obtain UL certification for our manufacturing plants. In order to obtain FDA approval, there is a high demand for documentation on testing, safety and quality standards, and performance for UL-certified plants. UL, IEC, and FDA have extensive documentation requirements to ensure the safe production of medical devices and to operate safely in medical environments. Regulators may not know exactly what the medical device does. However, they do understand the materials involved and provide guidelines to medical device manufacturers to produce their products in a manner that prevents failures that could result in personal injury or death. The goal is to ensure that no accidents occur. If an error occurs, these guidelines can also help the OEM and its supply chain track the root cause to prevent the error from occurring again. In order to properly track responsibilities, these regulators require complete documentation from equipment manufacturers and lithium battery manufacturers.
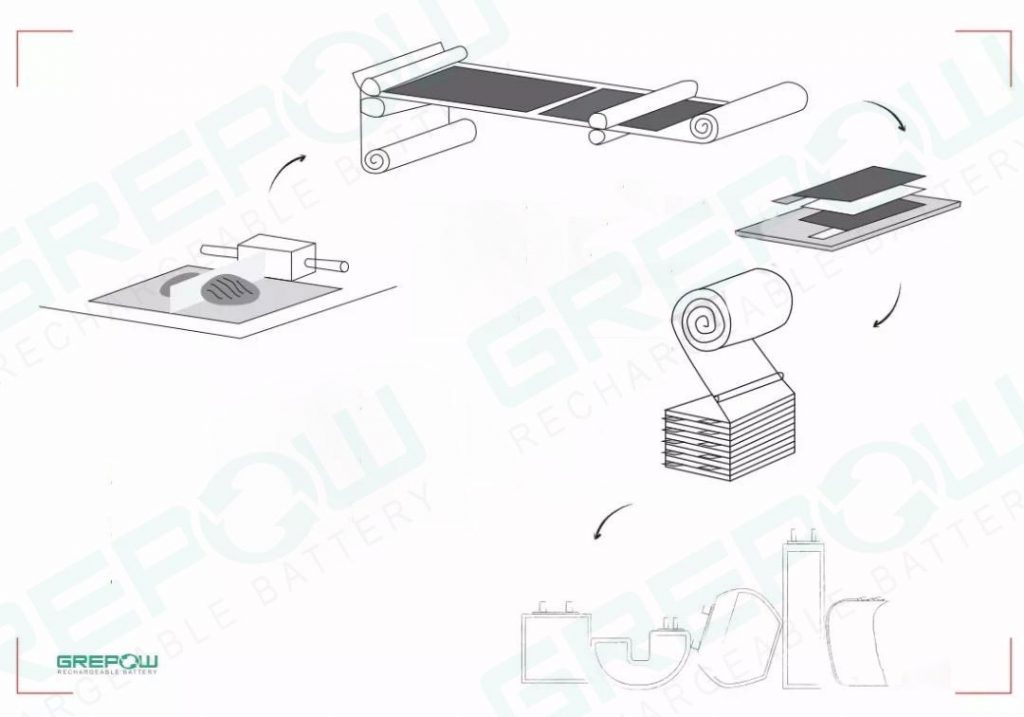
The design solution for the medical devices power requirements
GREPOW has been designing and manufacturing lithium battery pack solutions for medical applications for many years. The expertise we have gained enables our experts to find the right lithium chemistry formula and meet the specific needs of portable medical devices with a well-designed Smart Battery Management System (BMS). With a wealth of experience, medical device developers should now work with GREPOW as their professional medical lithium battery manufacturer instead of trying to develop their own battery solutions. We focus on the technical challenges of integrating battery power in a way that balances performance and safety in the best possible way. Our medical product lines are also diverse. We can support all medical devices with battery voltage requirements from 3.7V to 60V DC and capacities from 450mAh to 80Ah. OEMs and other medical device developers should seek professional advice or cooperation advice from GREPOW before taking the next step. For more details on how GREPOW designs the best solution for the power requirements of medical devices, please see our custom battery solution page.

GREPOW medical battery in the design phase
The design of the GREPOW medical battery completely solves the OEM safety problem. The standards we use in the development of medical batteries consider the worst-case scenario of abusing lithium batteries in any operating environment. E.g:
Medical equipment on fire: The mechanical design of the GREPOW medical battery allows the shape of the battery pack to be changed to prevent explosions, which can cause debris and damage.
The battery BMS is designed to be redundant and meets all hypothetical safety issues for UL inspectors.
Pass the high-temperature aging test, low-temperature aging test
Batch processing test with long batch processing
Drop and impact test
The physical destructive penetration test
These tests were conducted to evaluate the design of the GREPOW medical battery to understand how the GREPOW medical battery performs in protecting users in the event of any form of damage or environmental disaster. We can say with certainty that our battery design has passed these tests before it is produced.
Medical battery production at UL-certified factories
In order to comply with all medical certificates, lithium batteries must be produced by a UL-certified factory. From chemical production to battery pack assembly and final testing, lithium batteries must be produced in UL-certified plants. In the process of applying for FDA approval, the requirements for testing, safety and quality standards, and performance documentation for UL certified plants are high. Any medical device manufacturer should not purchase lithium batteries from a factory that is not UL listed because they will not receive FDA approval.

GREPOW's factories have passed UL certification for our medical battery product line. As noted above, UL, IEC, and FDA have extensive documentation requirements to ensure the safe production of medical devices and safe operation when used in a medical environment. We actively support OEM customers' applications by obtaining all the documentation required for regulatory approval.
GREPOW Medical Battery Features
1. Meets UL / IEC / UN safety guidelines
2. Prepare to provide quality and safety documentation to support OEM FDA applications
3. Safety design for overcharge/discharge protection
4. Safety design for overcurrent protection
5. High-temperature performance and safe design for protection
6. The unique mechanical design prevents injury in catastrophic conditions
7. Accelerate equipment development schedule (accelerate market launch)
Custom battery solutions to meet the medical application's needs
GREPOW is committed to using clean energy technologies to promote sustainability and create a better world. We plan to develop high-security, high-quality batteries for medical applications. Our medical batteries can be customized to integrate your creativity and meet specific needs. With over 20 years of customer service experience, Grepow has developed a very complete service system, specifically tailored for our customers, which helps us better understand your needs in the first step of our communication, in a highly time-efficient way. If you are interested in our customized battery, please don't hesitate to contact us at any time! Email: info@grepow.comGrepow Website: https://www.grepow.com/
Related Articles
-

Thin Film Lithium-ion Battery Vs Lithium-ion Battery: What’s the Difference?
2024-08-16 -

Prescription vs OTC Hearing Aids: What's the Difference?
2024-08-09 -

Continuous Glucose Monitors Battery Choose Guide
2024-08-02
Related products
-

Pouch Ultra Narrow Lipo Battery
-

Pouch Round Lipo Battery
-

Pouch Curved Lipo Battery
-
Pouch Ultra Thin Lipo Battery